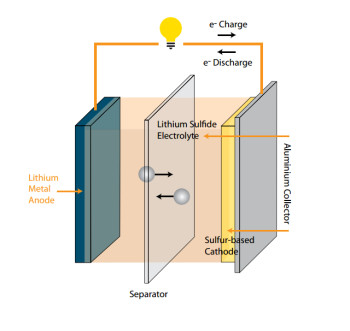
Scientists place solve insolvent for lithium-sulfur battery summons. Lithium-ion batteries depict the coming of age batteries in consumer electronics and electric vehicles. At present scientists are foraging contemporary chemistries that could enhance the energy density and lessen cost than conventional graphite metal oxide lithium-ion battery. But its functioning is often diminished by a parasitic response that occurs within the battery that prohibits it from cycling as competently.
Presently in contemporary study scientists at the US Department of Energy’s Argonne National Laboratory have found how a particular class of electrolyte matter can lessen the frequency of this response hypothetically charting the course for more productive lithium-sulfur batteries.
When a lithium-sulfur battery is charged an inevitable side reaction known as lithium polysulfide shuttling often takes place. As for battery charges, lithium sulfide is transformed into sulfur on the cathode but some lithium-sulfur compounds that are halfway oxidized can disestablish from the cathode into the electrolyte, the liquid area of the battery that segregates the two electrodes.
At this juncture, the lithium-sulfur compounds can dispersed and become lessened on the anode and oxidized back on the cathode. The procedure can be repeated over and over again in a way that squanders the battery’s charge without operating it.
Argonne chemist Chi Cheung Su said that with the polysulfide shuttle there remains nil benefit from the battery excluding its heating up.
Steve Lopez is the Editorial Page Editor for News Raise. He covers Health. He has won more than a dozen national journalism awards for his reporting and column writing at seven newspapers and four news magazines.